Measurement error and resolution in quantitative stable isotope probing: implications for experimental design
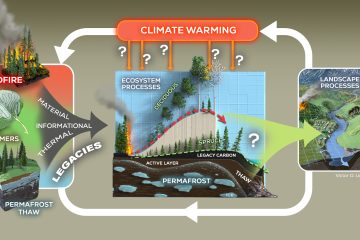
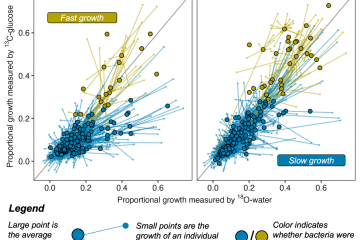
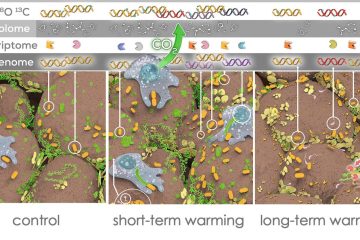
Quantitative stable isotope probing (qSIP) estimates the degree of incorporation of an isotope tracer into nucleic acids of metabolically active organisms and can be applied to microorganisms growing in complex communities, such as the microbiomes of soil or water. As such, qSIP has the potential to link microbial biodiversity and biogeochemistry. As with any technique involving quantitative estimation, qSIP involves measurement error; a more complete understanding of error, precision and statistical power will aid in the design of qSIP experiments and interpretation of qSIP data. We used several existing qSIP datasets of microbial communities found in soil and water to evaluate how variance in the estimate of isotope incorporation depends on organism abundance and on the resolution of the density fractionation scheme. We also assessed statistical power for replicated qSIP studies, and sensitivity and specificity for unreplicated designs. We found that variance declines as taxon abundance increases. Increasing the number of density fractions reduces variance, although the benefit of added fractions declines as the number of fractions increases. Specifically, nine fractions appear to be a reasonable tradeoff between cost and precision for most qSIP applications. Increasing replication improves power and reduces the minimum detectable threshold for inferring isotope uptake to 5 atom%. Finally, we provide evidence for the importance of internal standards to calibrate the %GC to mean weighted density regression per sample. These results should benefit those designing future SIP experiments, and provide a reference for metagenomic SIP applications where financial and computational limitations constrain experimental scope.Importance One of the biggest challenges in microbial ecology is correlating the identity of microorganisms with the roles they fulfill in natural environmental systems. Studies of microbes in pure culture reveal much about genomic content and potential functions, but may not reflect an organism’s activity within its natural community. Culture-independent studies supply a community-wide view of composition and function in the context of community interactions, but fail to link the two. Quantitative stable isotope probing (qSIP) is a method that can link the identity and function of specific microbes within a naturally occurring community. Here we explore how the resolution of density-gradient fractionation affects the error and precision of qSIP results, how they may be improved via additional replication, and cost-benefit balanced scenarios for SIP experimental design.